
Chemists began exploring bismuth compounds well over a century ago, searching for metals that could outperform toxic heavy metals in industrial and pharmaceutical settings. Bismuth isooctanoate first turned up as a promising option in the mid-to-late 20th century when environmental regulations started to tighten. Lead and tin had been the go-to catalysts for drying paints and curing polymers—both created real environmental headaches. The discovery of bismuth's low toxicity and decent catalytic capabilities pushed research teams to try bismuth isooctanoate in these roles. The shift from organotin and lead driers used in alkyd paints to bismuth-based driers reflects a broader cultural move toward greener industries and products. The steady pace of new patents and technical improvements over the past three decades shows this compound didn’t just fill a gap—it helped set new standards for safer, smarter chemistry.
Bismuth isooctanoate belongs to the broad class of carboxylates—metal complexes formed from fatty acids. In day-to-day conversation, the product often carries names like bismuth octoate, or just Bi-2EH, shorthand for “2-ethylhexanoate.” Companies market it in two forms: as a clear, yellowish to pale amber liquid, or as a solid, depending on concentration. It moves quickly through both lab supply warehouses and the back rooms of industrial facilities, typically packaged in metal or HDPE drums, kept as a key additive for coatings, plastics, and some elastomers.
Bismuth isooctanoate stands out among metal carboxylates for its high metal content (16–28% bismuth by weight). At room temperature, it remains pourable, which helps in handling. The liquid emits a faint, somewhat oily odor—not nearly as strong or unpleasant as similar tin-based products. Solubility gives it another advantage. Formulators easily dissolve it in organic solvents, resins, and many plasticizers, making it a practical choice for complex industrial mixes. Thermal stability proves sufficient for most paint curing and polyurethane manufacture. Boiling point sits beyond 250°C, which puts it out of range for evaporation losses at normal process temperatures. Bismuth's trivalent state stays steady, which means fewer unwanted side reactions or catalyst breakdowns even under extended heat and exposure.
Technical data sheets from reputable manufacturers spell out the essentials: bismuth content, acid number, specific gravity, and viscosity. For instance, a typical liquid concentrate contains 28% bismuth and has a specific gravity in the range of 1.1–1.3 at 25°C. Labels must follow global hazard codes (GHS/CLP), highlighting both environmental impact and personal health precautions. European and North American regulations ask for unique product identifiers, hazard pictograms, and detailed handling and disposal advice. Shelf life usually runs at least one year if sealed tight and stored away from moisture and sunlight. The technical focus in these documents lands on maintaining integrity from production through application, which serves both safety and performance.
Manufacturers prepare bismuth isooctanoate by reacting bismuth(III) oxide (Bi2O3) with 2-ethylhexanoic acid under controlled temperature—rarely exceeding 180°C. The process requires a mild organic solvent, careful stoichiometry, and efficient agitation. Side reactions must be avoided, so teams monitor the release of water as a byproduct and often use vacuum stripping to remove traces of moisture and unreacted acid. The routine isn’t complicated, but achieving high purity means following standard operation protocols, avoiding iron or copper catalysts that could contaminate the batch. Every step adds up to a clean, homogeneous catalyst with low free acid content, desired bismuth concentration, and an expected shelf life.
In the lab or the plant, bismuth isooctanoate typically acts as a Lewis acid, catalyzing esterification, transesterification, and polyurethane formation. The trivalent bismuth coordinates with oxygen atoms of carboxylic acid groups, activating them toward nucleophilic attack. In paint drying applications, it speeds up the cross-linking of unsaturated oils and resins. Some chemists tweak its ligands, swapping isooctanoic acid with longer-chain or branched carboxylic acids to modulate activity or solubility. Derivatives exist for specialty applications—these include bismuth neodecanoate and bismuth naphthenate, which offer higher solubility in non-polar matrices at the cost of a slightly different catalytic profile.
Ask three suppliers for bismuth isooctanoate, and you'll hear three versions: bismuth(III) 2-ethylhexanoate, bismuth octoate, and Bi-2EH. In industrial shorthand, old-timers might call it “bismuth drier” or “non-toxic octoate catalyst.” Specific product numbers often include the bismuth loading (e.g., “BIO-28%”). These names speak to regional history—Europe and North America stick with “isooctanoate,” East Asia favors “octoate.” Global sales teams often add a commercial catchphrase hinting at “green” or “heavy metal free,” because end-users look for regulatory and safety reassurance.
Companies rank bismuth isooctanoate as much safer than conventional lead and tin additives, but safe doesn’t mean risk-free. Skin and eye irritation can happen after direct contact, so workplace standards recommend gloves, splash goggles, and ventilation in blending or repackaging areas. Spills clean up with universal absorbents, then the waste enters the hazardous waste stream—not the regular trash. Regulatory authorities like OSHA and the EU REACH system keep close watch on potential cumulative effects, pushing manufacturers to keep detailed logs, offer up-to-date safety data sheets, and monitor workplace air continuously for vapor. Training programs keep workers from treating it casually. From an industrial hygiene perspective, these steps protect both people and the reputation of “green” chemistry in the wider community.
Paint and coatings production relies heavily on bismuth isooctanoate as a drier, especially for architectural alkyds and industrial enamels. It replaced many toxic catalysts after the 1990s, making it easier for companies to sell to Western Europe and North America without hitting regulatory snags. The polyurethane foam industry counts on it as a gelling and blowing catalyst for flexible and rigid foams—particularly useful in automotive seat cushions and building insulation. Beyond those two hotspots, it features in PVC stabilizer packages, certain adhesives, and some niche silicone curing applications. Film and sheet manufacturers, seeking better regulator compliance, choose bismuth isooctanoate to avoid residual heavy metals in final products. I’ve even seen it pop up in some antimicrobial polymer experiments, tapping bismuth’s mild biocidal action when blended into plasticized vinyls.
Academic interest zoomed in on bismuth isooctanoate about 20 years ago, fueled by both green chemistry trends and the search for better polyurethane catalysts. Chemical engineering labs explore custom ligand modifications—tailoring activity, solubility, and resistance to hydrolysis for different uses. Analytical groups push for improved trace detection and speciation techniques, tracking the fate of bismuth in effluent streams and recycled resins. Most studies agree on its lower cytotoxicity compared to mercury, tin, or lead equivalents, and that’s pushed some researchers to look at medical-grade bismuth isooctanoate for short-term implant coatings or wound dressings. The practical focus now turns toward using renewable feedstocks in its synthesis, and maximizing bismuth recovery from industrial scrap to close the loop on raw material use.
Toxicologists approach bismuth isooctanoate with cautious optimism. Standard tests in rodents show low acute toxicity by ingestion, though repeated or high-dose exposure can stress the kidneys and liver. The compound breaks down to release 2-ethylhexanoic acid and bismuth cations, both of which carry modest risks in large volumes but little reproductive or mutagenic harm in regular occupational use. Environmental scientists run soil and water studies, keeping an eye on how bismuth derivatives move through sludge and coastal runoff. Local authorities in strict jurisdictions call for periodic effluent testing and ask manufacturers to confirm their product’s compliance with regional eco-toxicological benchmarks, especially where landfill or incineration disposal routes enter the picture. Compared to persistently toxic metals like cadmium or hexavalent chromium, bismuth isooctanoate remains a lower-risk option. Still, downstream users can’t afford to lose perspective: just because it’s “safer” doesn’t mean it’s safe in all settings.
Looking ahead, bismuth isooctanoate will see greater demand in paints, coatings, and flexible foams as health and environmental regulations press for further reduction of heavy metals. Some companies already test biodegradable isooctanoic acid sources and push for biobased process cycles, lowering the cradle-to-grave impact of these metal carboxylates. Nanotechnology teams study bismuth’s hybrid organic-inorganic systems for electronics, solar, and catalysis, hinting at new high-value technical markets. Automation and AI-driven chemical development promise to speed up the screening of derivative compounds, rooting out residues or optimizing performance for next-generation polymers or specialty elastomers. If oversight keeps pace and recycling scales up, bismuth isooctanoate stands a good chance of anchoring future “green” manufacturing in both legacy industries and the new energy/materials sectors.
Bismuth Isooctanoate doesn’t show up on supermarket shelves, but it plays a bigger role in everyday products than most people realize. This compound helps make paints, varnishes, and coatings better and safer. Industry workers, myself included, care a lot about chemicals in paints, so the shift away from heavy metals like lead and tin matters. Serious health problems, including kidney and nerve issues, have been tied to those older chemicals. Bismuth-based alternatives came in because they avoid most of those risks.
Paint factories run into complex cures. They need something that triggers drying without messing up the finish or leaching toxins into the air. That’s the main value of Bismuth Isooctanoate — it helps coatings dry hard and clear. Oil-based paints and some specialty lacquers rely on it. Over the years, I’ve seen manufacturers choose this route because it cuts down on heavy metals while still making sure performance stays strong. Regional regulations in places like the EU encourage the move, and product recalls tied to hazardous chemicals push the point further.
Bismuth Isooctanoate checked two important boxes: lower toxicity and enough performance. For paint shop workers, this makes a real-world difference. I’ve walked through plants where solvent-heavy air left a weird itch in my throat. By using less hazardous driers and stabilizers, the job feels safer. Research shows bismuth compounds break down more safely in the environment than lead salts do. The risk of water and soil pollution goes down, which local agencies track and care about.
There’s an economic angle, too. Switching to safer options sometimes raises material costs but decreases expenses related to environmental cleanup or worker health claims. Smaller shops, which can’t afford big legal problems or accidents, watch these changes closely. As rules keep updating, safer additives like Bismuth Isooctanoate become a must instead of a luxury.
Bismuth isn’t just for paint buckets. Some bismuth compounds help fight stomach ulcers or infections, and research is ongoing. Bismuth Isooctanoate’s use in medicine hasn’t reached the same scale, but chemists haven’t closed the door. A company I worked with once explored its use as a catalyst for pharmaceutical reactions. Getting new uses off the ground means studying safety, breakdown in the body, and waste disposal. Responsible scientists publish this research openly.
Constant improvement defines modern industry. Choosing safer, smarter components reduces headaches later. Bismuth Isooctanoate doesn’t solve every environmental or health worry, but its adoption marks steady progress away from old, high-risk chemicals. Experts at universities and safety labs keep testing, making sure the industry doesn't backslide. Turning toward these alternatives shows companies listen to both regulators and the people painting their houses and furniture.
Using Bismuth Isooctanoate isn’t a cure-all. It takes coordination between manufacturers, regulators, and workers. Factories need routine training so crews handle new ingredients correctly. Pay attention to updated safety data sheets, which often explain how to keep exposures low. Companies should invest in air filters and personal gear even with safer compounds in place. On the regulatory front, tighter tracking of paint and coating formulas blocks slip-ups that used to be common.
Everyone gains when cleaner technologies find their way into standard practice. Bismuth Isooctanoate stands as a solid case study: science moves forward, companies adapt, and everyday people end up with safer products at home and on the job.
Bismuth Isooctanoate often shows up in industrial labs and polymer processing lines. A lot of us working in materials science or manufacturing see it as an alternative metal catalyst. Some newer processes have shifted to bismuth-based compounds to move away from toxic heavy metals like tin. Even though it poses lower environmental toxicity, thinking this makes it “safe” for direct handling can open the door to trouble. Skin exposure, inhalation of vapors, or splashes in the eyes can still cause irritation or longer-term sensitivity.
No big industrial accident drove this home for me — just watching a colleague rinse out a beaker, get distracted, and end up with irritation on his wrists for days. Even seasoned pros slip up. I always reach for a fresh pair of impervious gloves when weighing or transferring Bismuth Isooctanoate. Nitrile does the job in small labs, though heavier-duty rubber gloves have saved my skin on days with heavy mixing.
I never work with volatile organometallics near anything that would trap fumes. Good ventilation can’t be a box checked on a form; you want visible airflow or, better yet, a tested fume hood. Bismuth compounds carry low acute toxicity, but inhaling any fine mist or vapor can tickle airways and lead to headaches or worse. Opening sealed bottles in a dead space builds risk.
Goggles feel like overkill when weighing powder, but I once saw someone get a fine bloom of catalyst settle on their eyelashes. Any splash risk should push you to grab the goggles. For larger transfers, face shields combine with goggles for a better barrier.
Labeling seems like basic housekeeping until you see unlabeled vials on a cluttered rack. One mistake, one unmarked bottle swapped at shift change, and a small risk turns serious. I make it a point to write the full material and concentration since some bismuth mixtures look alike. Proper storage matters more than people realize — keep containers sealed tight in a dry, cool spot, away from acids and bases that might kick off a reaction. Double-checking compatibility protects both your inventory and the people unloading the shelf later.
Someone in every lab should know the drill for a bismuth isooctanoate spill: contain with absorbent material, scoop with minimum dust, and bag waste for hazardous disposal. Ordinary cleaning rags or dry sweeping can churn up dust and risk inhalation for others later. Wiping down surfaces with industrial wipes followed by soap and water keeps the workspace safe for the next team. Most labs keep a “spill kit” within arm’s reach for these occasions.
Reading a safety data sheet offers information, but nothing beats training with actual materials. Colleagues new to bismuth catalysts should shadow a veteran through their first few runs — hands-on guidance sticks better than bullet point lists. Supervisors should run refresher sessions and highlight near-misses to remind everyone these compounds deserve respect.
Working with new materials like Bismuth Isooctanoate can lull people into a false sense of safety just because it’s less notorious than others. For me, the right approach blends careful PPE use, sharp labeling, quick cleanup skills, and real-world mentoring. Taking these steps in stride builds a culture that looks out for everyone, from the intern prepping samples to the technician monitoring inventory.
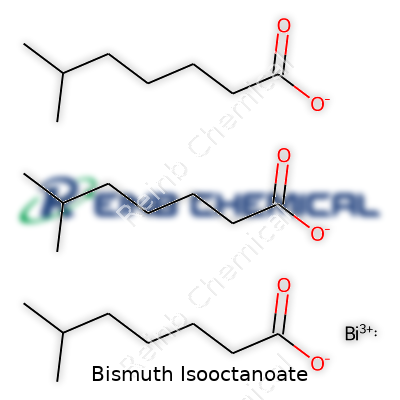
Bismuth has a reputation for making medicines gentler on the stomach. Pairing it with isooctanoic acid (a derivative of caprylic acid) creates bismuth isooctanoate, a compound sometimes found in pharmacy shelves and chemical supply catalogs. Chemically, it’s made by swapping out the hydrogen in isooctanoic acid with bismuth ions. You’ll see the formula as Bi(C8H15O2)3. Each molecule chains three isooctanoate ions to a single bismuth atom.
Understanding the formula isn’t about showing off knowledge. For me, it’s like having a good recipe on hand. If you know what went into your meal, you feel more secure about eating it. In medicine, clinicians and pharmacists track every atom. If a patient reacts badly, knowing the full chemical makeup points everyone in the right direction. There are stories from the 1980s where even small tweaks to bismuth compounds led to health scares. Clear formulas keep misunderstandings in check.
Bismuth isooctanoate pops up in digestive medicines and sometimes in lubricating compounds for machines. Reliable production depends on predictable chemistry. If a supplier doesn’t stick to the right formula, batch quality dips fast. Standards fall, and then side effects crop up. That exact formula—Bi(C8H15O2)3—anchors the quality controls that protect both patients and machine operators. My own work in chemical logistics showed me how paperwork tied to formulas prevents disasters. No pharmacist (or factory worker) should worry about what’s really inside that drum.
Stories from medicine remind us: bismuth isn’t just another metal. After cases of bismuth toxicity shook Europe decades ago, regulators started tightening formulas and forcing clearer labels. The stakes go beyond one hospital: loose handling makes recalls tricky and lawsuits almost inevitable. The US Food and Drug Administration looks for exact formulas in pre-market review, and European agencies match this vigilance. From the chemists I’ve spoken to, they say supply chains have gotten safer only when everyone agreed on strict chemical definitions.
Clear chemical formulas help everyone—patients, doctors, chemical suppliers, and manufacturers—work from the same blueprint. When everyone gets the same information, confusion shrinks. As more counterfeit products enter global markets, strict disclosure makes tracing trouble sources easier for health professionals and regulators alike. If there’s a rash of negative drug reactions, formula transparency speeds up the solution. That single string of elements and numbers—Bi(C8H15O2)3—delivers all the background needed to spot fakes, correct errors, and move forward with confidence.
Grabbing a bottle of Bismuth Isooctanoate and tossing it on a shelf isn’t smart or safe. This compound doesn’t show up in most people’s lives, but for those who work in labs or production lines, storage tells you a lot about risk control and good practice. Picture a sunny, open warehouse with bottles stacked shoulder-high. That’s the wrong setting. Bismuth Isooctanoate, like other metal-organic compounds, demands controlled conditions — not just for safety but to extend its usefulness.
Direct sunlight and heat might not seem like enemies until you realize what they can do. Heat can change the chemical stability, mess with shelf life, and worst case, lead to dangerous decomposition. I’ve seen labels peel off plastics that sat near south-facing windows, leaving everyone guessing what’s really inside. For Bismuth Isooctanoate, a cool, dark corner wins every time. Most storage guides recommend temperatures below 30°C (86°F). It’s not about babying the chemical. It’s about not letting its properties cause headaches down the line, or worse, accidents.
Forget those giant open drums. Bismuth Isooctanoate performs best kept in tightly sealed containers. Exposure to air invites moisture, which threatens purity and makes spills tough to clean up. I learned the hard way that poorly closed jugs lead to sticky residue and stubborn odors. Strong caps make a difference, and clear labeling means no guessing games for the next person. Some labs go for glass over plastic to keep things inert and resist leaching from certain plastics, especially when the product stays on the shelf for months.
Chemical compatibility counts every time. Store Bismuth Isooctanoate far away from acids, alkalis, and any substance that could spark a reaction. I’ve seen outdated storage rooms crammed with all sorts of chemicals. That cross-contamination risk is real; one mishap can lead to expensive cleanup or injuries. An isolated cabinet, preferably lockable and ventilation-friendly, keeps things safer for everyone.
Even careful storage doesn’t rule out accidents. Absorbent mats on shelving, ready-to-grab spill kits, and easy access to emergency showers and eyewash help deal with surprises. In one manufacturing site, I saw the difference between theory and practice: a simple spill kit saved the day after a loose cap fell off. Nobody wants to rush a cleanup with lab coats, not when a few sensible storage steps could have prevented trouble in the first place.
Proper storage of Bismuth Isooctanoate isn’t just about ticking a box. It protects workers, saves money, and respects environmental regulations. Following safety data sheet advice pays off, but so does keeping seasoned workers in the loop about risks. Regular checks and a culture of reporting small leaks catch problems early and show that good habits matter more than fancy systems.
Safer storage means setting a standard. It’s about taking small steps — shelving in cooler areas, giving chemicals their own neighborhood, sealing them up, and labeling well. It all adds up. The people who use and handle Bismuth Isooctanoate count on these practical choices. There’s no shortcut worth taking if long-term safety, quality, and compliance are on the line.
For years, the chemical industry relied heavily on tin and lead-based catalysts in processes like polyurethane production. Folks like me who’ve spent time in labs know that these metals come with a steep environmental bill. Tin, in particular, turns up in wastewater streams and can linger for decades. Lead doesn’t just disappear either; people worry about it for good reason. Regulators have started to apply real heat on factories discharging these substances. The push isn’t just coming from activists—insurers and savvy consumers are raising their voices, too.
Developers searching for alternatives have zeroed in on bismuth-based compounds. Bismuth isotooctanoate stands out as a candidate for replacing tin and lead in many catalytic reactions. Why? Bismuth doesn’t build up in plants and animals, so people and wildlife don’t end up with it in their systems. Its lower toxicity profile makes a real difference, especially for folks working in production lines or communities close to industrial sites.
Swapping out catalysts isn’t just about ticking off compliance boxes: the new pick needs to get the job done. Bismuth isotooctanoate has shown strong results in applications like foam production and coatings. Manufacturers like that it gives predictable results without the clean-up headaches clinging to tin systems. It hands off less nastiness to air and water, helping companies keep emissions below legal limits. Parents, workers, and regulators all want fewer health scares down the road.
No chemical comes risk-free. Bismuth itself comes from the earth, usually as a by-product of mining lead, copper, and tungsten. Bismuth mining produces fewer hazardous tailings than most tin mining. That’s good news, though transportation and refining still burn energy and add some environmental drag. Some mining operations lag behind best practices, so real-world differences depend on supply chain choices. Traceability matters; the greenest catalyst won’t offset a dirty mine.
Every catalyst eventually needs disposal. Bismuth isotooctanoate breaks down into less mobile forms that stay trapped in sediments and soils, unlike tin compounds that slip into water tables and food webs. Landfills and municipal incinerators struggle far less with bismuth, lowering cleanup bills for local governments. Policymakers can set tighter discharge rules without worrying about hurting manufacturers that switch to bismuth.
People want products with less environmental baggage, but swapping catalysts isn’t just a snap decision for most factories. Adopting bismuth isotooctanoate works best with open communication between researchers, regulators, and industry leaders. More publicly available data on life-cycle impacts will help buyers and local officials steer production in healthier directions. Sharing practical experience about handling and blending the compound can cut down on mistakes. Investments in recycling and recovery of bismuth from industrial waste streams will shrink the environmental footprint even further.
Watching the shift in industry practice up close, I’ve learned that small shifts in chemical recipes can ripple outward. Bismuth isotooctanoate isn’t perfect, but choosing it over legacy options lets us produce the goods we rely on with less regret. The switch is not just for eco-label bragging rights—it’s about real people and places that live with the consequences. I’d rather see my colleagues and neighbors working around bismuth than the heavy metals of the past.
| Names | |
| Preferred IUPAC name | 2-Ethylhexanoic acid;bismuth(3+) |
| Other names |
Bismuth(3+) 2-ethylhexanoate Bismuth(III) 2-ethylhexanoate Bismuth isooctanoic acid salt Bismuth octoate |
| Pronunciation | /ˈbɪz.məθ aɪ.səʊˈɒk.tə.neɪt/ |
| Identifiers | |
| CAS Number | 68186-91-4 |
| Beilstein Reference | 1841015 |
| ChEBI | CHEBI:81713 |
| ChEMBL | CHEMBL2107231 |
| ChemSpider | 21564313 |
| DrugBank | DB09481 |
| ECHA InfoCard | 09ad7116-b267-4e02-bcf3-3b4e6b9c4e92 |
| EC Number | 301-152-6 |
| Gmelin Reference | 87806 |
| KEGG | C18606 |
| MeSH | D001662 |
| PubChem CID | 166873 |
| RTECS number | UJ4375000 |
| UNII | RV8H4U60L2 |
| UN number | UN3334 |
| CompTox Dashboard (EPA) | DTXSID0054592 |
| Properties | |
| Chemical formula | C23H47BiO4 |
| Molar mass | 567.7 g/mol |
| Appearance | Light yellow to yellow liquid |
| Odor | Odorless |
| Density | 1.16 g/cm3 |
| Solubility in water | Insoluble |
| log P | 1.51 |
| Vapor pressure | Negligible |
| Basicity (pKb) | 7.02 |
| Magnetic susceptibility (χ) | -1.67e-4 |
| Refractive index (nD) | 1.570 |
| Viscosity | 200-600 cP |
| Dipole moment | 0.00 D |
| Pharmacology | |
| ATC code | A02BX05 |
| Hazards | |
| Main hazards | May cause respiratory irritation. |
| GHS labelling | GHS07, GHS08 |
| Signal word | Warning |
| Hazard statements | H317: May cause an allergic skin reaction. |
| Precautionary statements | P261, P273, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 113 °C |
| Lethal dose or concentration | LD50 (oral, rat) > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 >5000 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 85% |
| Related compounds | |
| Related compounds |
Bismuth subgallate Bismuth subsalicylate Bismuth subcarbonate Bismuth oxychloride Bismuth tribromophenate |